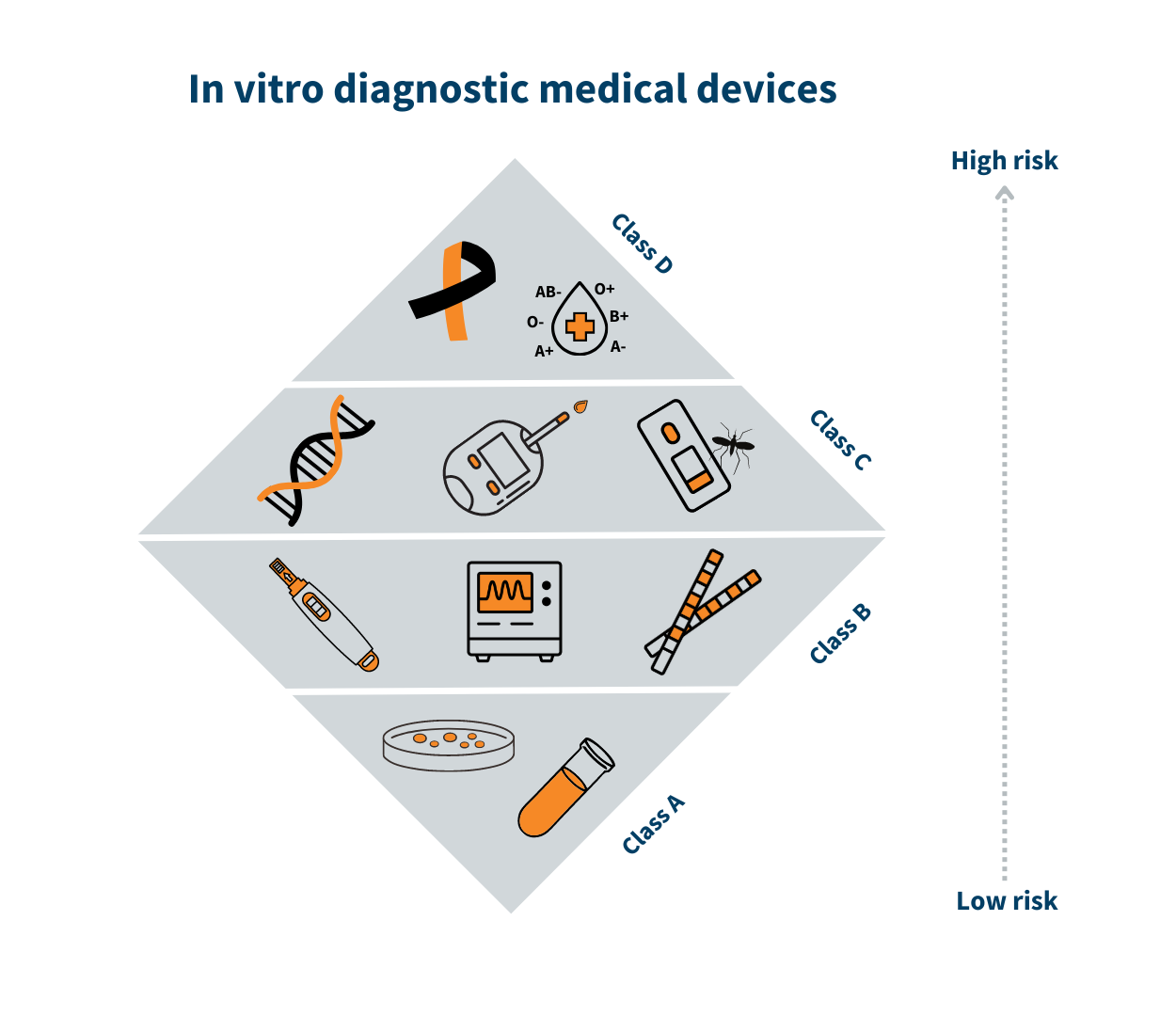
Recommended structure for approving and monitoring medical devices,... | Download Scientific Diagram

Competent Authority, Notified Body, ISO Registrar: How Each Role Functions in the Medical Device Industry

EU Finalizes New Medical Device Regulations (MDR) which update the regulatory framework for the marketing of devices and IVDs in Europe – Catchtrial
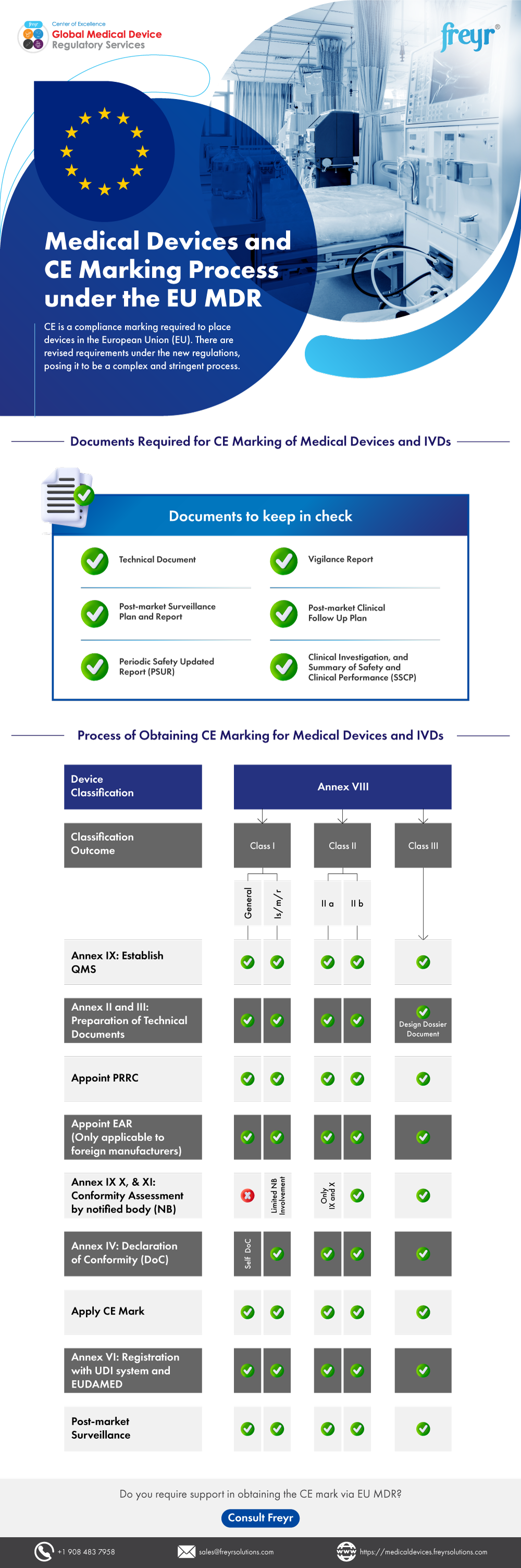




%20On%20EU%20Notified%20Bodies.jpg)
%20On%20EU%20Notified%20Bodies.jpg)
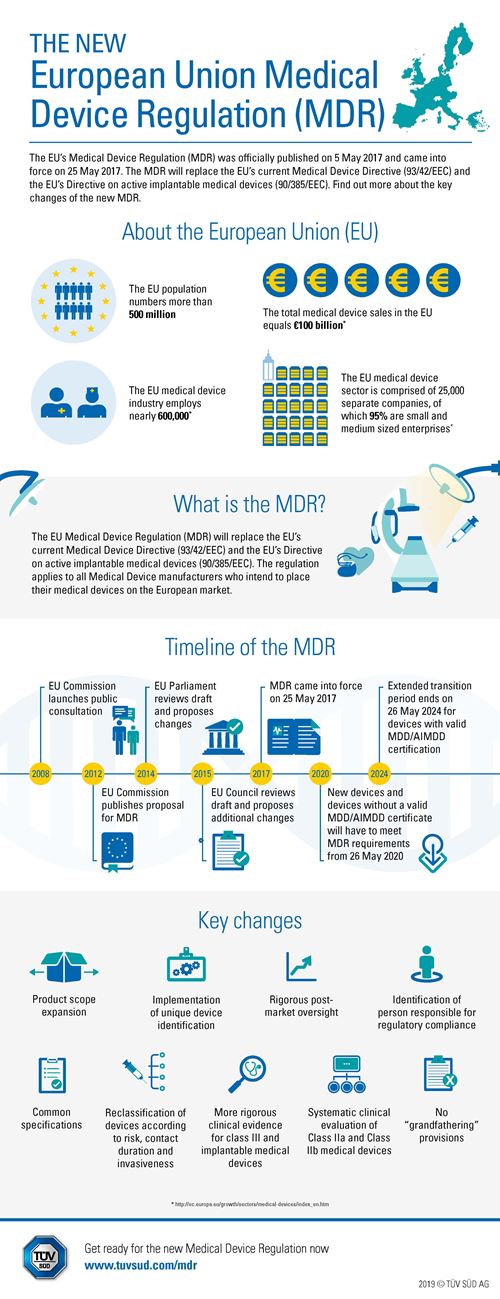

/tuv-rheinland-ivdr-visual-1-en.png)