Current Oncology | Free Full-Text | Validation of a Patient-Reported Outcome Measure for Moist Desquamation among Breast Radiotherapy Patients

A review of criteria strictness in “Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials” - ScienceDirect
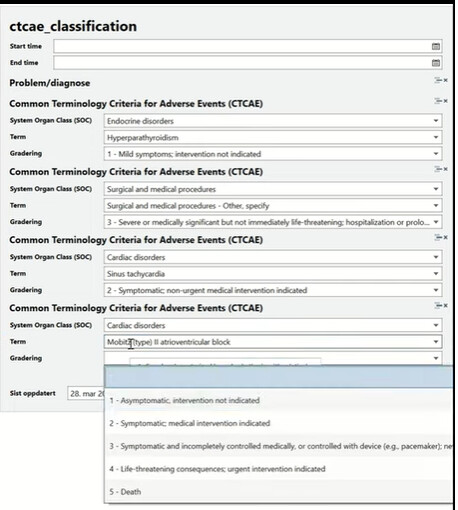
The National Cancer Institute's Common Terminology Criteria for Adverse... | Download Scientific Diagram

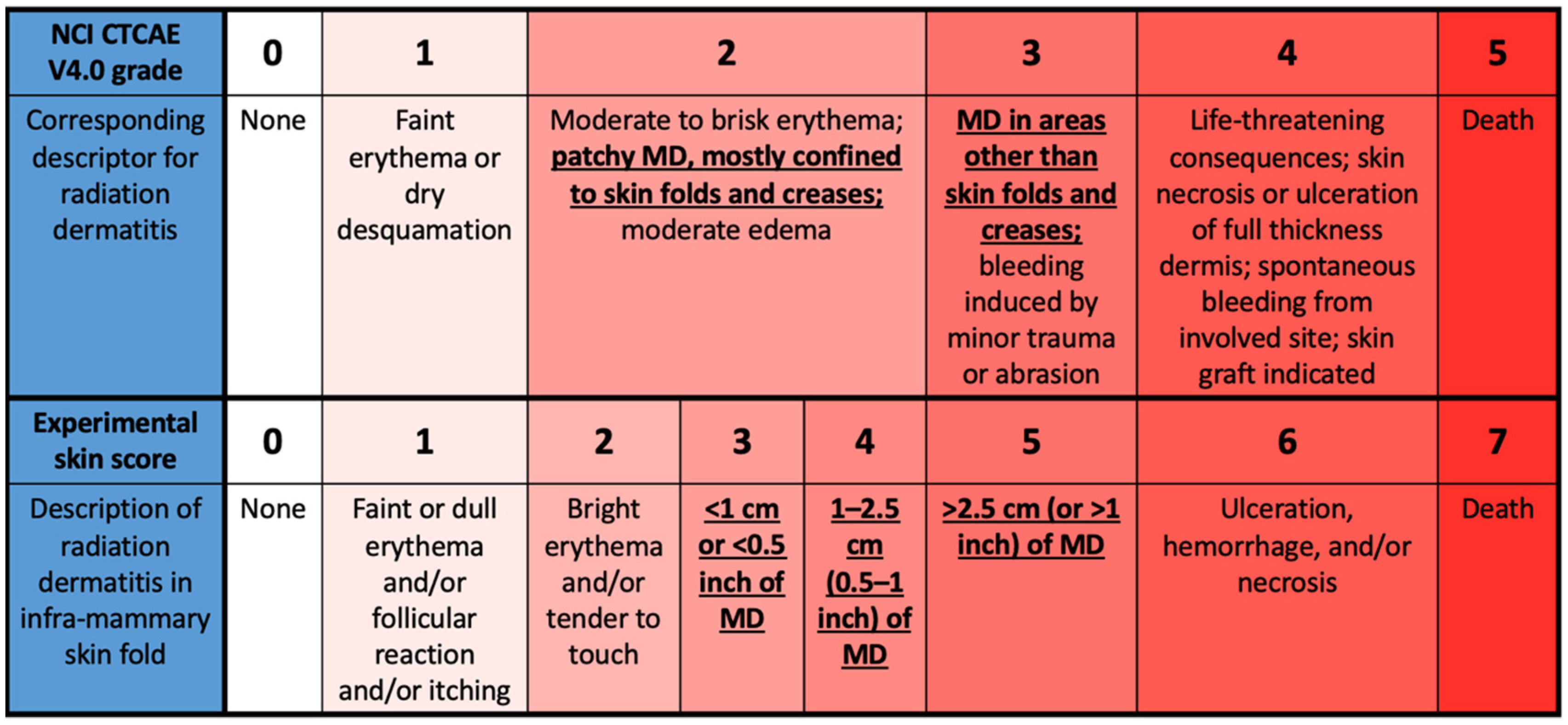
Toxicity grading scale in the common terminology criteria for adverse... | Download Scientific Diagram

Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) - Ethan Basch, Claus Becker, Lauren J Rogak, Deborah Schrag, Bryce B

Full article: Clinician and Patient Reporting of Symptomatic Adverse Events in Cancer Clinical Trials: Using CTCAE and PRO-CTCAE® to Provide Two Distinct and Complementary Perspectives

Use and misuse of common terminology criteria for adverse events in cancer clinical trials | BMC Cancer | Full Text

Composite grading algorithm for the National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) - Ethan Basch, Claus Becker, Lauren J Rogak, Deborah Schrag, Bryce B

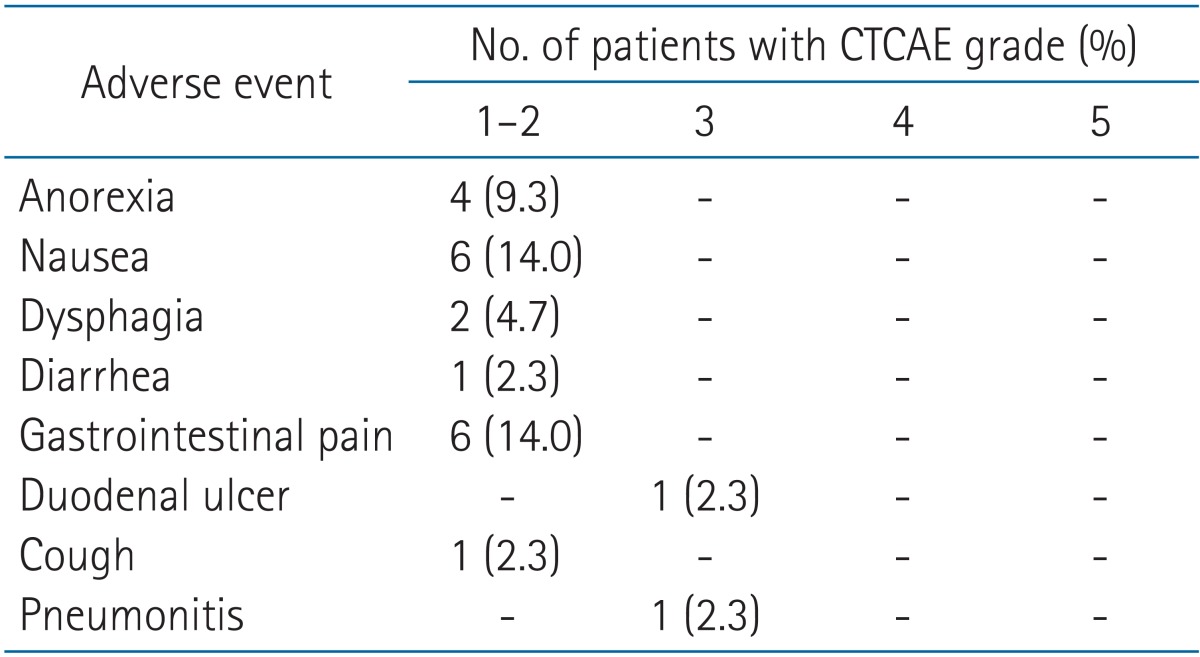
Common Terminology Criteria for Adverse Events (CTCAE) for diarrhea and... | Download Scientific Diagram

Establishing a common metric for patient-reported outcomes in cancer patients: linking patient reported outcomes measurement information system (PROMIS), numerical rating scale, and patient-reported outcomes version of the common terminology criteria ...

Veterinary cooperative oncology group – common terminology criteria for adverse events (VCOG‐CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats v1.1 - 2016 - Veterinary and Comparative Oncology - Wiley Online Library


![Grading scales of the CRS Penn Grading Scale [20] CTCAE v5.0 [80]... | Download Scientific Diagram Grading scales of the CRS Penn Grading Scale [20] CTCAE v5.0 [80]... | Download Scientific Diagram](https://www.researchgate.net/publication/334158848/figure/tbl2/AS:852086526844929@1580164667382/Grading-scales-of-the-CRS-Penn-Grading-Scale-20-CTCAE-v50-80-Lee-MDACC-16.png)


![Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF Ctcae 4.02 2009-09-15_quickreference_8.5x11[1] | PDF](https://image.slidesharecdn.com/ctcae4-022009-09-15quickreference8-5x111-120711021618-phpapp01/85/ctcae-402-20090915quickreference85x111-2-320.jpg?cb=1668437655)