Use and misuse of common terminology criteria for adverse events in cancer clinical trials | BMC Cancer | Full Text
Using the Skindex-16 and Common Terminology Criteria for Adverse Events to assess rash symptoms: results of a pooled-analysis (N0993) - Document - Gale Academic OneFile
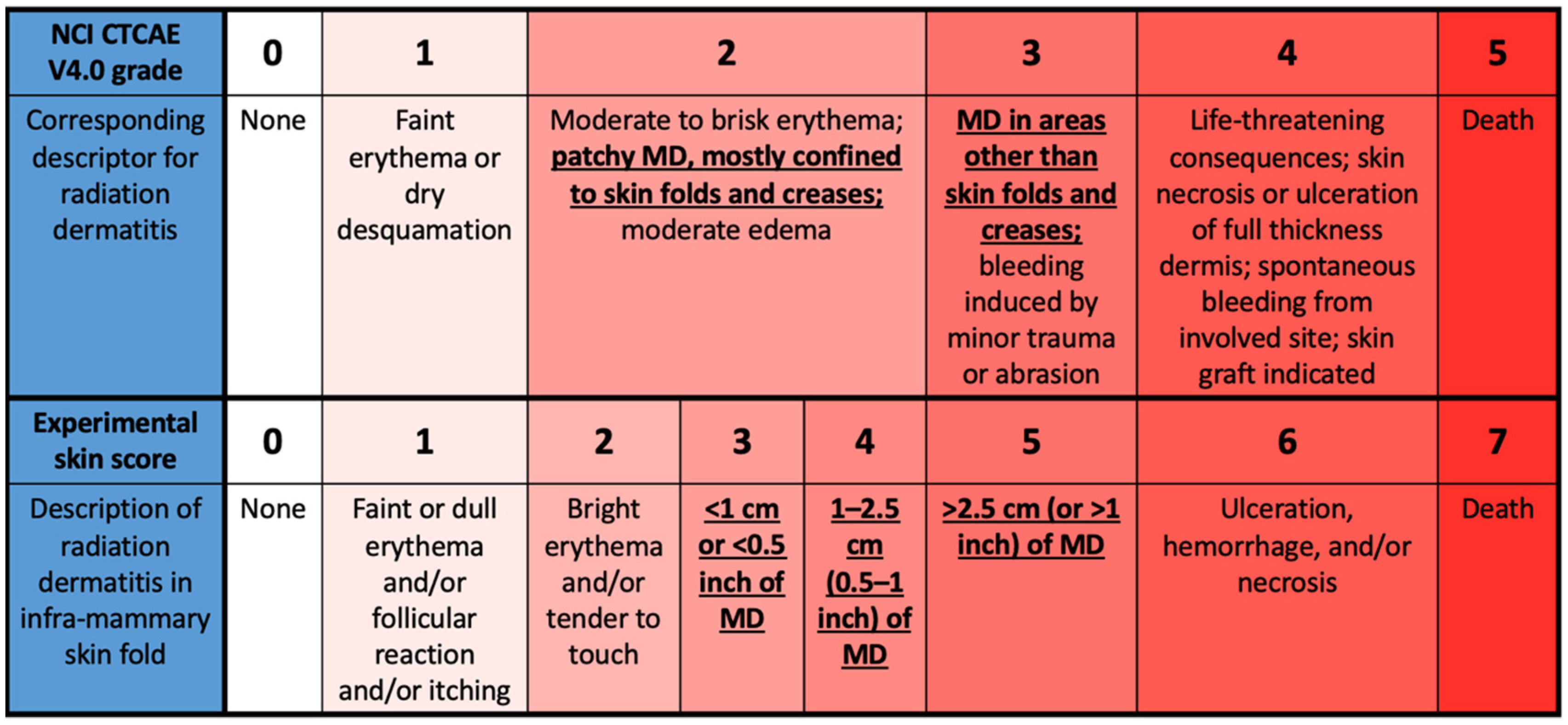
The National Cancer Institute's Common Terminology Criteria for Adverse... | Download Scientific Diagram
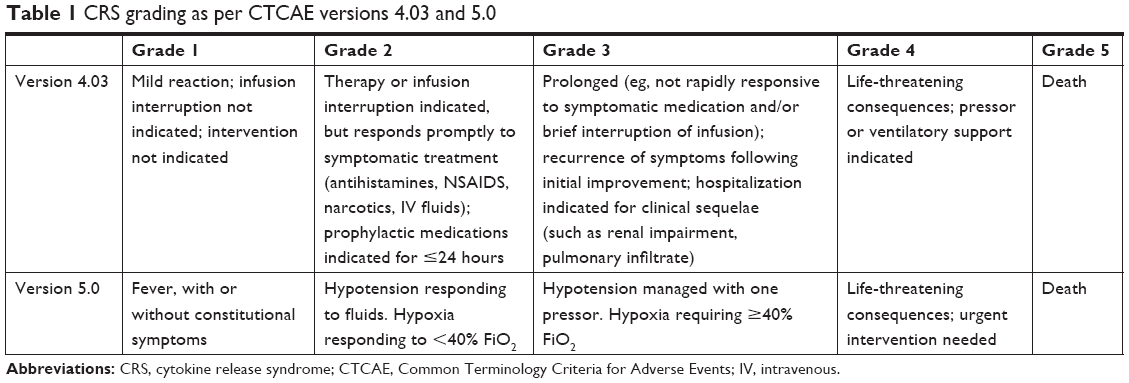
![PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/72d6066aa43136e48ae6226e3dba7e542a7a02de/5-Table1-1.png)
PDF] Patient-reported outcomes and the evolution of adverse event reporting in oncology. | Semantic Scholar
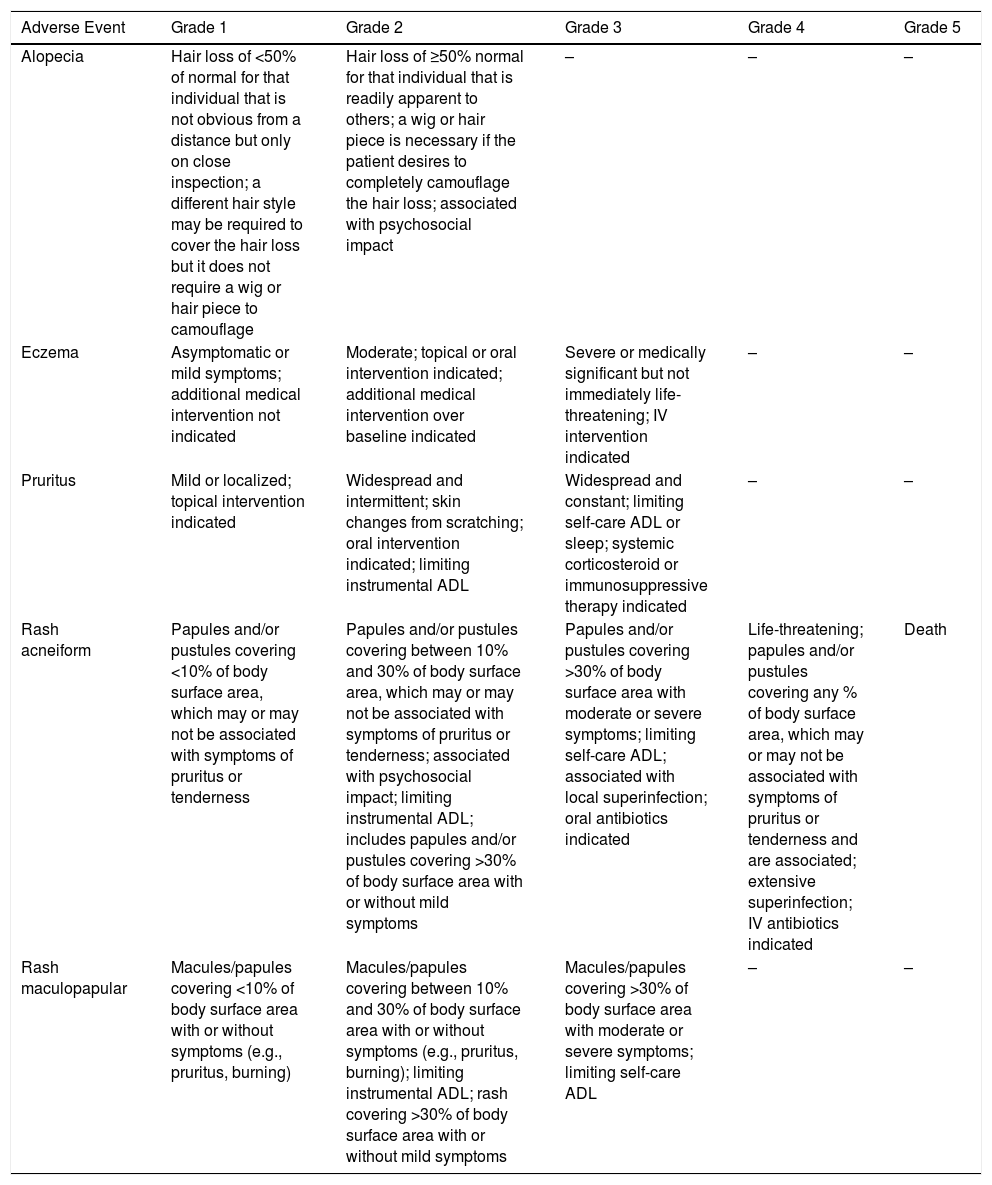
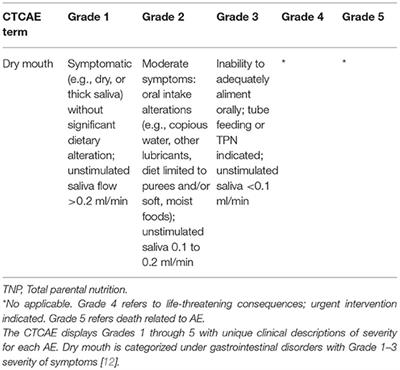
Using the Common Terminology Criteria for Adverse Events (CTCAE – Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies | Actas Dermo-Sifiliográficas

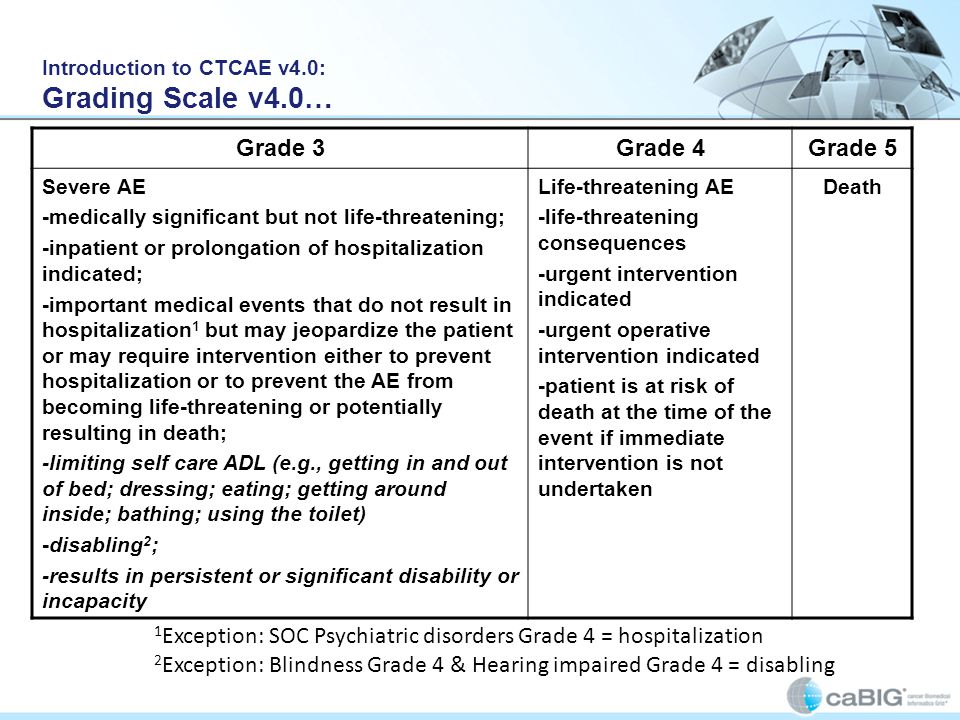
Table 4 from The use of and adherence to CTCAE v3.0 in cancer clinical trial publications | Semantic Scholar