Designation process of MDR/IVDR Notified Bodies - update · MDlaw – Information platform on European medical device regulations

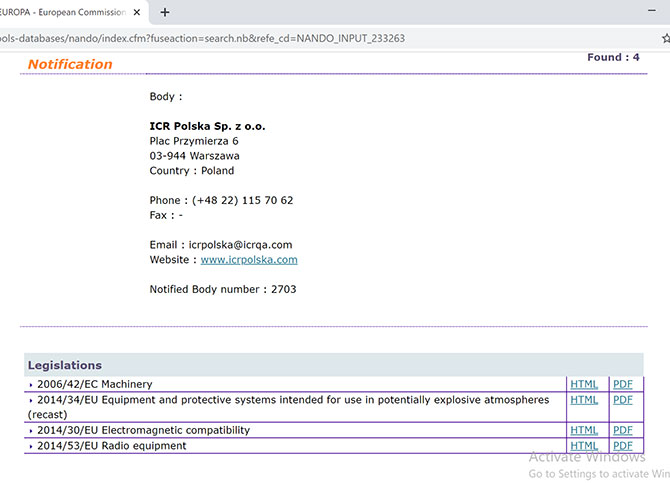
FIND OUT THE ECM ACCREDITATIONS ON NANDO, THE EU DATABASE OF NOTIFIED BODIES - Ente Certificazione Macchine

Mario Gabrielli Cossellu on LinkedIn: #notifiedbody #mdr #nando #ivdr #conformityassessment #regulations…

Which EU Notified Bodies Have Been “Designated” Under the MDR 2017/745 and IVDR 2017/746? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog
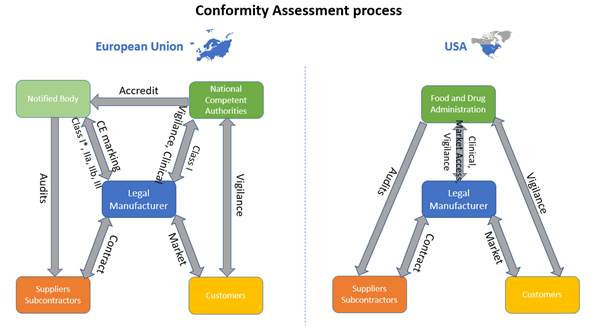
What are the principal differences between the conformity assessment process of a medical device in the USA and in the European Union? - Kvalito





![EU MDR Quality Management System [Role of an eQMS Software] EU MDR Quality Management System [Role of an eQMS Software]](https://www.simplerqms.com/wp-content/uploads/2022/11/eu-mdr-notified-bodies-database.jpg)