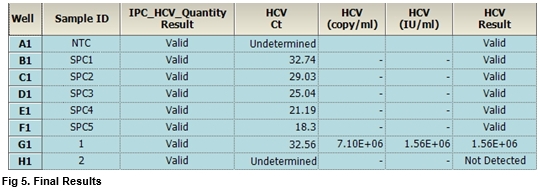
HCV RNA quantification in capillary dried blood spots with the Xpert® HCV Viral Load test for diagnosing chronic HCV infection, monitoring treatment and detecting reinfection
Rapporti ISTISAN 00/32 ISTITUTO SUPERIORE DI SANITÀ V Seminario di aggiornamento sull'epatite da virus HCV e nuovi virus pote

Monthly medians of HCV RNA and ALT levels in individuals with acute HCV... | Download Scientific Diagram
Servizio “Sanità, lavoro e politiche sociali” Codice sito: 4.10/2022/25 Al Ministero della salute Gabinetto gab@postacert.s

Evaluation of the cobas® HCV test for quantifying HCV RNA in dried plasma spots collected using the cobas® Plasma Separation Card - ScienceDirect
Servizio “Sanità, lavoro e politiche sociali” Codice sito: 4.10/2022/25 Al Ministero della salute Gabinetto gab@postacert.s